On-Demand Webinar:
EU MDR & the New Requirements for Hazardous Substances in Medical Devices
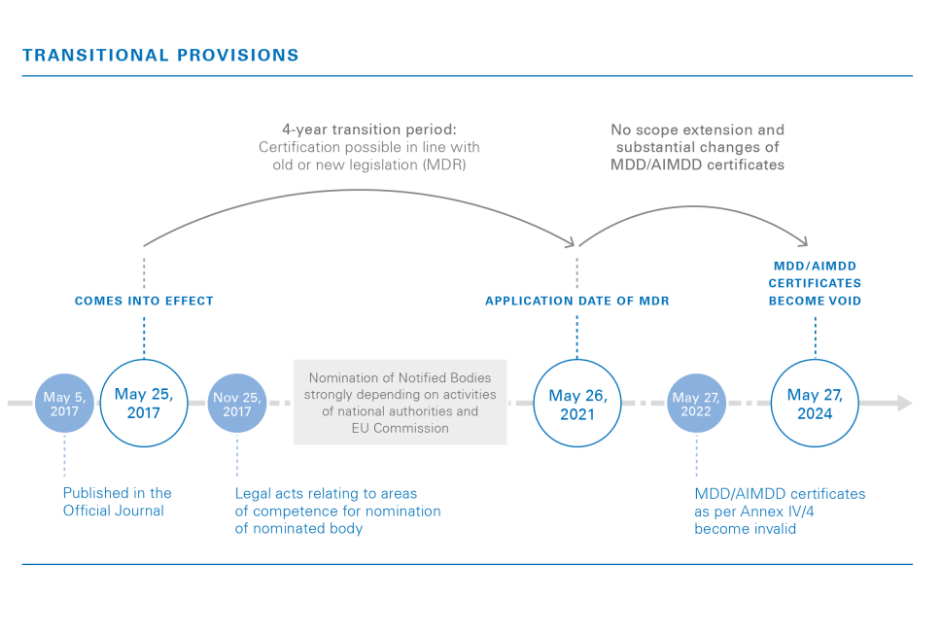
With the EU MDR entering into effect in less than a year, it’s more important than ever for manufacturers to understand compliance for their medical devices. Within the medical device industry, supplier declarations have been proven to be unreliable. Often times, suppliers do not accurately know what their products contain, they lack proper education, and unfortunately, some suppliers have been known to outright lie on their declarations of conformity. However, when it comes down to it, it’s manufacturers who bear the burden of proving their medical device is safe, not the supplier.
As a medical device manufacturer, here are some questions to consider:
As a medical device manufacturer, here are some questions to consider:
- Have you considered the restrictive list of CLP substances required for “Invasive” materials in your Medical and IVD devices?
- How will you prove your medical devices contain no substances classified as Carcinogenic, Mutagenic, or Toxic for Reproduction (CMR 1A and 1B)?
- How will you ensure your medical device upholds the highest possible standard of quality?
- How can you avoid worst-case scenarios such as a non-conformance, FDA recall, legal litigation and/or possible removal from the market?